"Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Size And Forecast by 2031
The comprehensive research report provides an in-depth overview of the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market, covering its size, scope, demand, and growth prospects. Companies aiming to thrive in this competitive landscape can benefit from the actionable insights and strategic guidance offered in the report.
The Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market has shown consistent growth in recent years, with its size increasing significantly due to expanding demand across industries. Industry statistics highlight a robust rise in value, driven by the adoption of innovative products and solutions. Companies are leveraging emerging opportunities to enhance their market share and revenue. The scope of the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market spans various sectors, making it a lucrative space for stakeholders. Insights from market research underscore the pivotal role of industry trends in driving this growth.
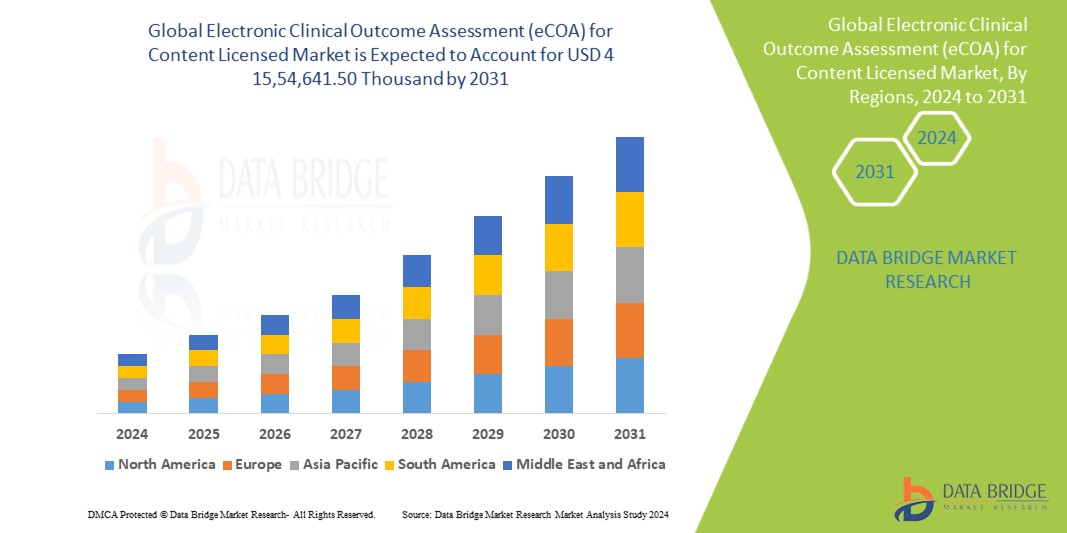
Data Bridge Market Research analyses that the Global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market which was USD 474708.5 Billion in 2023 is expected to reach USD 1554641.5Thousand by 2031 and is expected to undergo a CAGR of 14.40% during the forecast period of 2023 to 2031
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Which are the top companies operating in the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market?
The global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market study presents a detailed analysis of the industry, focusing on key trends, market dynamics, and the competitive landscape. It highlights leading companies in the market, examining their strategies and contributions to market share. Additionally, the report offers insights into the Top 10 Companies in Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market in the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market, including their business strategies, financial performance, and overall market position.
**Segments**
- Based on content type, the global electronic clinical outcome assessment (eCOA) for content licensed market can be segmented into Patient-Reported Outcome (PRO), Clinician-Reported Outcome (ClinRO), Observer-Reported Outcome (ObsRO), and Performance Outcome (PerfO). PROs are directly reported by the patient without interpretation by anyone else, ClinROs are reported by healthcare providers, ObsROs are reported by observers or caregivers, and PerfOs are assessments of a patient's ability to perform certain tasks or activities.
- On the basis of product type, the market can be segmented into Web-based eCOA, Clinician-Administered eCOA, and Patient-Administered eCOA. Web-based eCOA involves patients or clinicians entering data into an electronic system via a web interface, Clinician-Administered eCOA comprises assessments conducted by healthcare professionals using electronic devices, and Patient-Administered eCOA involves patients completing assessments on electronic devices independently.
- By end user, the market can be categorized into Hospitals, Clinics, Contract Research Organizations (CROs), Pharmaceutical & Biotechnology Companies, and Academic Research Institutes. Each end user segment has unique needs and requirements when it comes to utilizing electronic clinical outcome assessment solutions for clinical trials and healthcare research purposes.
**Market Players**
- Some of the key players operating in the global electronic clinical outcome assessment (eCOA) for content licensed market include Medidata Solutions, Inc., Oracle Corporation, eClinical Solutions LLC, ERT Clinical, CRF Bracket, and IQVIA. These companies offer a range of eCOA solutions designed to improve data collection, patient engagement, and overall clinical trial efficiency.
- Other significant market players include Parexel International Corporation, Bioclinica, YPrime, Anju Software, Inc., Kayentis, and Dassault Systèmes. These companies also play a vital role in providing contentThe global electronic clinical outcome assessment (eCOA) for content licensed market is experiencing significant growth and evolution, driven by the increasing adoption of digital technologies in healthcare research and clinical trials. The segmentation of the market based on content type offers a nuanced understanding of the different types of outcomes being assessed. Patient-Reported Outcome (PRO) segment provides direct insights from patients themselves, while Clinician-Reported Outcome (ClinRO) offers perspectives from healthcare providers. Observer-Reported Outcome (ObsRO) involves inputs from observers or caregivers, and Performance Outcome (PerfO) focuses on evaluating a patient's ability to perform specific tasks or activities. These diverse segments cater to various stakeholders involved in the assessment and collection of clinical outcome data.
Moreover, the segmentation based on product type highlights the diverse ways in which eCOA solutions are utilized in healthcare settings. Web-based eCOA systems enable efficient data entry via online interfaces, while Clinician-Administered eCOA solutions facilitate assessments conducted by healthcare professionals using electronic devices. Patient-Administered eCOA products empower patients to independently complete assessments, enhancing engagement and streamlining data collection processes. The flexibility offered by these different product types contributes to the overall effectiveness and user satisfaction with eCOA implementations.
The segmentation by end user further underscores the wide-ranging applications of eCOA solutions across various healthcare settings. Hospitals, clinics, Contract Research Organizations (CROs), Pharmaceutical & Biotechnology Companies, and Academic Research Institutes represent distinct end-user segments with specific requirements and preferences for electronic clinical outcome assessment tools. Tailoring eCOA offerings to meet the unique demands of each end user category is crucial for driving adoption and ensuring the successful integration of technology into clinical research and healthcare practices.
Moving on to market players, the global eCOA market is characterized by the presence of key companies that are at the forefront of developing innovative solutions for data collection and outcome assessment in clinical trials. Players such as Medidata Solutions, Inc., Oracle Corporation,**Market Players**
- Medidata Solutions, Inc.
- Oracle Corporation
- eClinical Solutions LLC
- ERT Clinical
- CRF Bracket
- IQVIA
- Parexel International Corporation
- Bioclinica
- YPrime
- Anju Software, Inc.
- Kayentis
- Dassault Systèmes
- ArisGlobal
- Clinical Ink
- Signant Health
- WIRB-Copernicus Group
The global electronic clinical outcome assessment (eCOA) for content licensed market is witnessing substantial growth driven by the escalating integration of digital technologies in healthcare research and clinical trials. The segmentation of the market based on content type provides a detailed understanding of the various outcomes being evaluated. The differentiation in segments such as Patient-Reported Outcome (PRO), Clinician-Reported Outcome (ClinRO), Observer-Reported Outcome (ObsRO), and Performance Outcome (PerfO) offers diverse perspectives and insights from patients, healthcare providers, observers, caregivers, and performance assessments.
Furthermore, the segmentation based on product type showcases the versatility of eCOA solutions in healthcare settings. Differentiating between Web-based eCOA, Clinician-Administered eCOA, and Patient-Administered eCOA demonstrates the varied approaches to data collection and assessment methodologies. Web-based solutions enhance efficiency, clinician-administered tools ensure professional oversight, and patient-administered products promote patient engagement and autonomy in the assessment
Explore Further Details about This Research Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Report https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Why B2B Companies Worldwide Choose Us for Revenue Growth and Sustainability
- Gain a clear understanding of the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market, its operations, and stages in the value chain.
- Explore the current market scenario and assess future growth potential throughout the forecast period.
- Strategize effectively for marketing, market entry, expansion, and business plans by analyzing growth factors and buyer behavior.
- Stay ahead of competitors by studying their business models, strategies, and prospects.
- Make data-driven decisions with access to comprehensive primary and secondary research.
Key Insights from the Global Global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market :
- Comprehensive Market Overview: A detailed examination of the global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market.
- Industry Trends and Projections: Analysis of historical data (2015 onward) and future growth forecasts, including compound annual growth rates (CAGRs).
- Emerging Opportunities: Identification of new market prospects and targeted marketing strategies.
- Focus on R&D: Insights into demand for new product launches and innovative applications.
- Leading Player Profiles: Detailed profiles of major market participants.
- Market Composition: Analysis of dynamic molecule types, targets, and key resources.
- Revenue Growth: Examination of global market revenue, segmented by key players and product categories.
- Commercial Opportunities: Analysis of sales trends, licensing deals, and co-development opportunities.
Regional Insights and Language Accessibility
- North America: United States, copyright, Mexico
- Europe: Germany, France, UK, Russia, Italy
- Asia-Pacific: China, Japan, Korea, India, Southeast Asia
- South America: Brazil, Argentina, Colombia, and others
- Middle East and Africa: Saudi Arabia, UAE, Egypt, Nigeria, South Africa
Understanding market trends at a regional level is crucial for effective decision-making. Our reports cater to diverse audiences by offering localized analyses in multiple regional languages. These reports provide tailored insights for specific regions, enabling businesses and stakeholders to access relevant information for informed strategies. By bridging communication gaps, we empower regional markets to thrive and grow. Access our reports in your preferred language for a personalized understanding of industry dynamics.
Japanese : https://www.databridgemarketresearch.com/jp/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Chinese : https://www.databridgemarketresearch.com/zh/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Arabic : https://www.databridgemarketresearch.com/ar/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Portuguese : https://www.databridgemarketresearch.com/pt/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
German : https://www.databridgemarketresearch.com/de/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
French : https://www.databridgemarketresearch.com/fr/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Spanish : https://www.databridgemarketresearch.com/es/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Korean : https://www.databridgemarketresearch.com/ko/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Russian : https://www.databridgemarketresearch.com/ru/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975
Email:- [email protected]"